Extractables and leachables are contaminants or impurities that are produced in pharmaceutical processes like manufacturing process, container closure system and packing material of drug products which affects directly on their purity and efficacy. Extractables and leachables testing is critical to identify and quantify of the theoretically harmful impurities. These impurities can migrate from pharmaceutical container closure systems to contaminate a drug product and then it may become a risk to a patient’s health and drug product’s quality issues.
What are extractables and leachables?
According to The FDA the two terms are defined as follows:
• Extractables are compounds that can be extracted from the container closure system when in the presence of a solvent.
• Leachables are compounds that leach into the drug product formulation from the container closure system as a result of direct contact with the formulation.
In May 1999, the U.S. FDA published a guidance document on Container Closure Systems for Packaging Human Drugs and Biologics. The FDA mandated that pharmaceutical manufacturers demonstrate the safety of materials used in manufacturing or production systems, container closure systems or drug delivery devices. Failure to establish material safety could result in failure to receive FDA approval for a product. A new guideline on the assessment and control of extractables and leachables (E&L) is proposed. ICH guidelines covering many aspects of impurities.
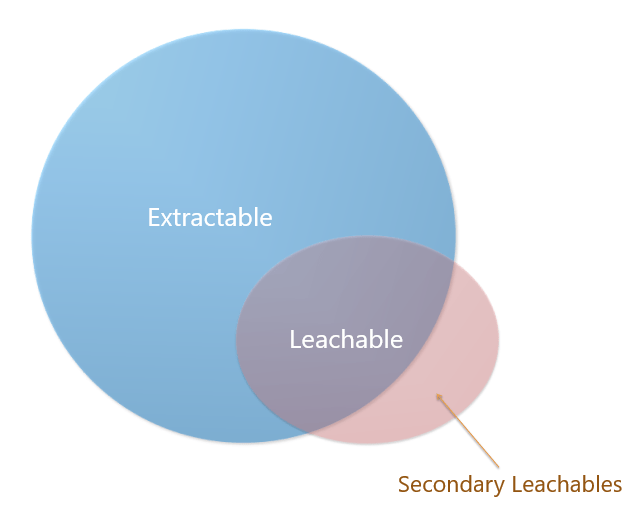
Difference between extractables and leachables
As we know that, leachables are a subset of extractables. They are both caused by either manufacturing processes or a lack of control of additives or agents used in the raw materials. Extractables are caused by extreme conditions and leachables can occur under normal conditions. The difference between extractables and leachables is that the leachables may always be present in sample storage container, and extractables may only be present after gamma irradiation has been applied to sterilize containers manufactured in a clean room.
Testing related to extractables and leachables
To ensure patient’s safety, the FDA requires all Drugs to be qualified to ensure that no chemicals transfer into the drug from its packaging or production. Such an analysis is called Extractables and Leachables Testing.
1. We at Chromak research provide services of testing extractables and leachables using various instruments including ICP-MS, GC-MS, LC-MS to quantify toxicological impurities at low level or concentration. .
2. We apply standard methods to identify and quantify of unknown organic and elemental impurities. We can also develop and validate new methods as per client’s needs. Our scientists also perform chemical testing of E&L impurities at Chromak Research e.g., pH, Conductivity, Total Organic Carbon etc.
3. Our well experienced team can conduct E&L (Extractables and leachables) analysis or testing in accordance with regional guidance and nationally or internationally recognized standards including GMP, USP guidance for E&L (USP chapters <665>, <1665>, <1663>, <1664>, and <1664.1>), European Medicines Agency (EMA) guidance, US Food and Drug Administration, FDA extractables and leachables guidance, Product Quality Research Institute (PQRI) extractables and leachables guidelines and ISO 10993-18 extractables and leachables guidelines (Part 18).
Chromak Research has fully equipped state of the art laboratory facilities and various techniques with ISO 9001:2015 and ISO/IEC 17025:2017. Our experts have many years of experience in focused analytical testing and toxicology assessment for extractable leachable impurities testing. We provide leachables method validation compliant to Good Manufacturing Practice (GMP) for use in GMP stability testing and storage programs and can support a wide range of closure or drug delivery systems
